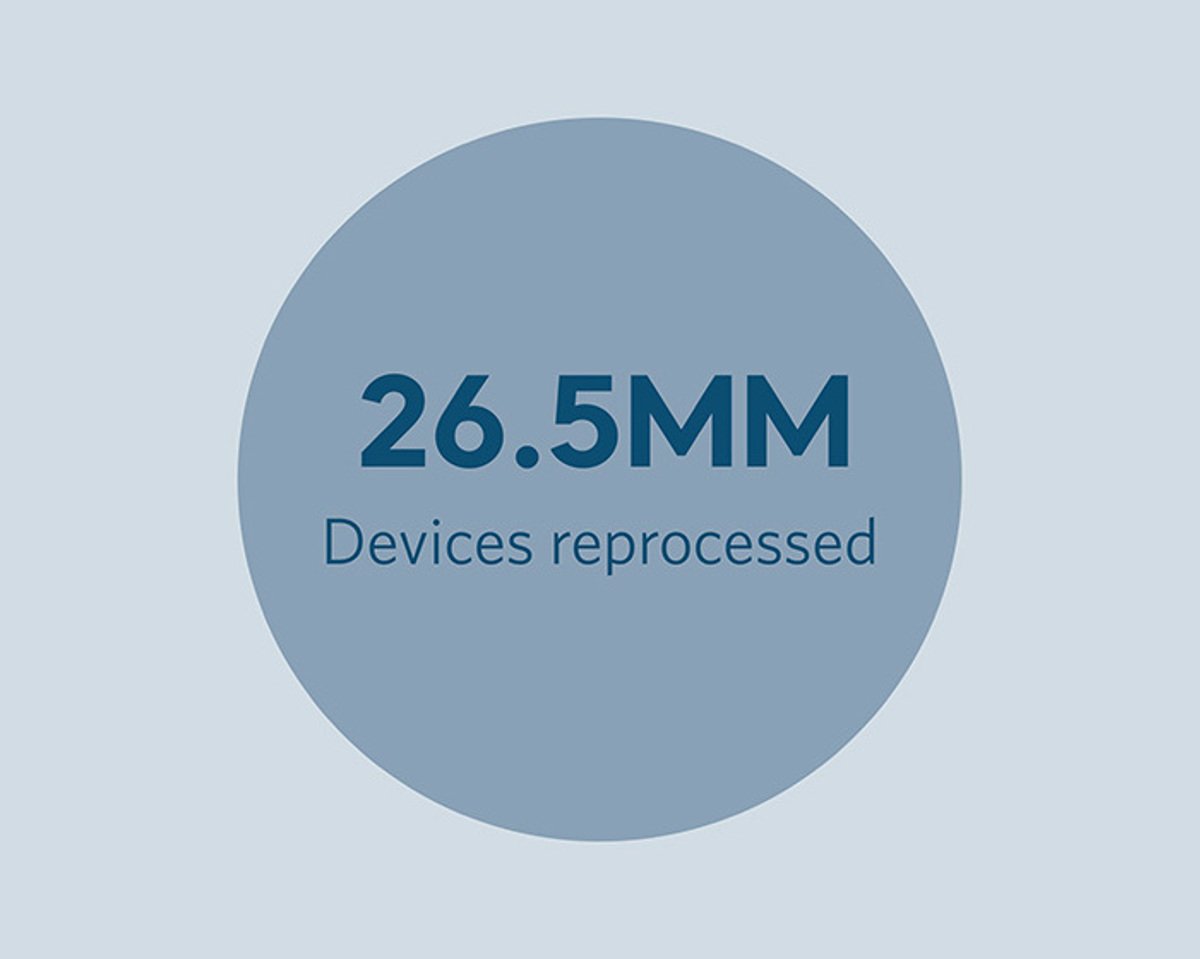
Arjo ReNu high-level disinfection (HLD) reprocessing uses a natural water-based process for the safe, non-toxic reprocessing of DVT garments, pulse oximeter probes, lateral transfer matts (such as Hovermatts®) and many other noncritical, noninvasive single use devices to maximize supply chain savings and waste elimination.

Our reprocessing solutions
Arjo ReNu high-level disinfection (HLD) reprocessing uses a natural water-based process for the safe, non-toxic reprocessing of DVT garments, pulse oximeter probes, lateral transfer matts (such as Hovermatts®) and many other noncritical, noninvasive single use devices to maximize supply chain savings and waste elimination.
The benefits of Arjo ReNu reprocessing for noninvasive medical devices

1. Cost-effectiveness
Experience significant savings over OEM devices and additional savings related to waste disposal and inventory reduction.
2. Environmental sustainability
Extend the lifecycle of devices and produce less landfill waste with green processes that produce zero harmful emissions.1–4

3. Safety
Promote the wellbeing of your patients and staff with reprocessed devices that look, feel, and function as brand new.

4. Service
As with every solution Arjo provides, our reprocessing program is backed by personalized support at every step of the process.
Watch on-demand
Be part of the sustainability solution and a leader in transformation to a circular economy. Watch the on-demand video now to learn how we can make a difference by “Bringing the Health Sector into the Circular Economy” with one of the world’s most authoritative reprocessing industry experts, Daniel J. Vukelich, Esq., President & CEO of the Association of Medical Device Reprocessors (AMDR).

AMDR member
We are a member of the Association of Medical Device Reprocessors (AMDR) which is the global trade association consisting of members of the commercial single-use device reprocessing and remanufacturing industry. AMDR represents regulated, commercial reprocessing, promotes reprocessing as an important healthcare strategy that helps hospitals and healthcare providers increase quality, reduce costs and improve patient care, and protects the interests of its members in regulation, legislation and standard-setting world-wide.

* Cost-savings and waste diversion are estimates, and have been calculated based on industry averages.
Devices reprocessed by Arjo ReNu

DVT Compression Garments
Manufacturers:
- Arjo
- Cardinal Health
- Currie Medical Specialities
- DJO
- Medline
- Zimmer Bioment

Air-Assisted Lateral Transfer Mats
Manufacturers:
- Arjo
- HoverTech
- Stryker/Sage

ECG Leads
Manufacturers:
- Advantage Medical Cables
- Cardinal Health
- Drager
- Philips

Pulse Oximeter Probes
Manufacturers:
- Masimo
- Nellcor

Pressure Infusion Bags
Manufacturers:
- CareFusion
- Ethox
- Merit Medical

Slings
Manufacturers:
- HoverTech

Chair Fall Sensor Pads
Manufacturers:
- Posey
- Stanley

Tourniquet Cuffs
Manufacturers:
- Stryker
- Zimmer Biomet

Arjo ReNu: a recycling solution that reduces the climate impact of healthcare
Medical consumables comprise a large proportion of the total climate impact of healthcare. As such, recycling solutions provide significant opportunities to reduce emissions.

Talk to us
Learn more about our Arjo ReNu reprocessing solutions by speaking with an Arjo expert, who will respond to your request in a timely manner.

MDSAP certified
We are committed to assuring our processes meet or exceed all recommendations for reprocessing by the FDA, CDC and other regulatory agencies. ReNu is proudly ISO 13485 and MDSAP certified.
To learn more about Arjo ReNu reprocessing, please click on the links below

About us
Reprocessing can be achieved by safely disinfecting noncritical, noninvasive single-use medical devices so they can be used again.

Instructions for Use
Download Instructions for Use files.

HLD pasteurization process
Arjo ReNu offers a variety of service options for hospitals to consider when implementing a reprocessing program for noncritical, noninvasive medical devices.

Benefits of HLD pasteurization
Our top priority is to provide a safe reprocessing solution for patients, clinicians and the planet.

Products that can be reprocessed
Arjo ReNu reprocesses a number of noncritical, noninvasive medical devices, from ECG leads to DVT garments.

Frequently asked questions (FAQs)
Here you'll find answers to commonly asked questions. If you don't see what you need, please contact us with your question.
References:
- Office of Device Evaluation, US Food and Drug Administration. Section 510(k) Summary No. K031559. November 12, 2003.
- Office of Device Evaluation, US Food and Drug Administration. Section 510(k) Summary No. K051227. April 6, 2006.
- Office of Device Evaluation, US Food and Drug Administration. Section 510(k) Summary No. K093658. January 14, 2010.
- Office of Device Evaluation, US Food and Drug Administration. Section 510(k) Summary No. K121145. June 12, 2012.