Symbliss®
What bathing should feel like
A feeling beyond clean. This is what bathing should feel like. Immersed in water, enjoying one of life’s essential pleasures. Explore Symbliss®, our latest innovation in bathing.

Dedicated to
Empowering Movement

5 solutions that support single-handed care
The term single-handed care simply refers to a means of safely transferring an individual with the correct equipment and appropriate number of carers.
In some care environments, there have long been ‘informal working practices’ automatically requiring the need for two caregivers when assisting with patient handling activities. However, in recent years there has been a definite shift towards the provision of single-handed care, especially within long-term care settings.
How can you improve on an icon?
Be the first to know about our latest innovation in multifunctional floor lifts.

Introducing MyArjo: Your portal to Arjo tools and services
MyArjo is a personalised web portal providing access to Arjo tools and services with one secure login.
Sign up to get MyArjo access and begin your journey to Empower Movement today.
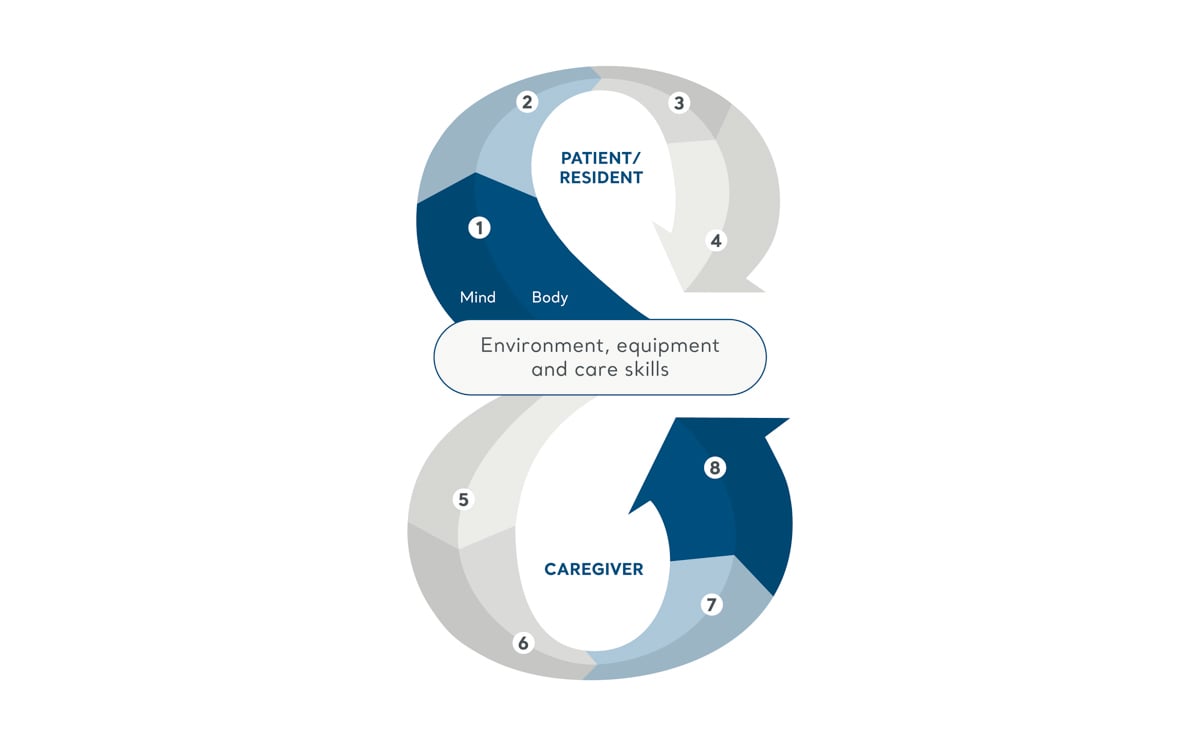
The Positive Eight Philosophy
Our care philosophy explains how facilitating mobility can catalyse a chain of benefits impacting quality of life, caregiver satisfaction, operational efficiency, and financial outcomes.
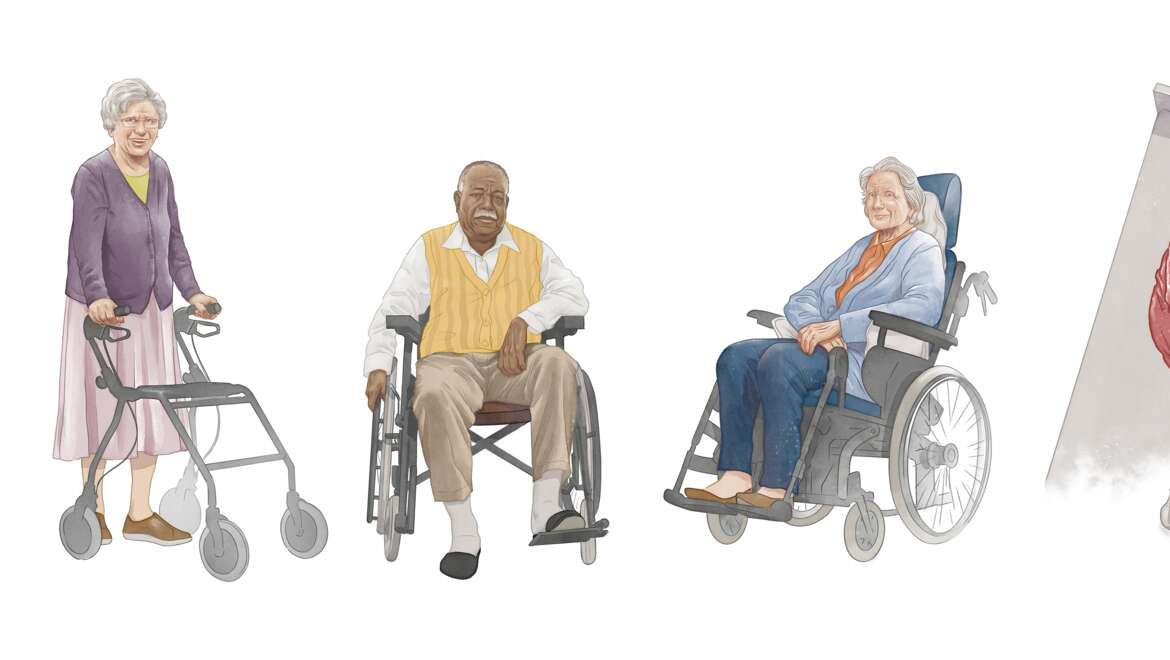
Mobility gallery
Based on 5 levels of mobility from A to E, the Arjo Mobility Gallery provides a basis for understanding how an individual can contribute to their mobility, as well as how to safeguard caregivers from injuries.

Arjo MOVE
Rooted in clinical evidence and driven by your facility data, Arjo MOVE programs arm you with the tools and knowledge to reach your clinical and operational goals.
6,500
employees globally
1957
Arjo was founded
100+ countries
where Arjo sells products and services
Empowering Movement
is at the heart of everything we do
Preventing the impact of immobility
Promoting greater mobility with dignity
Protecting the wellbeing of caregivers
Optimizing costs and efficiencies in care
Arjo’s interim report January-June 2025
Freeing up time to focus on what matters most: how Arjo supports better healthcare delivery
Arjo announces date for 2025 Q2 and conference call
Arjo issues bond under MTN program
Arjo establishes MTN program



