Auralis Plus
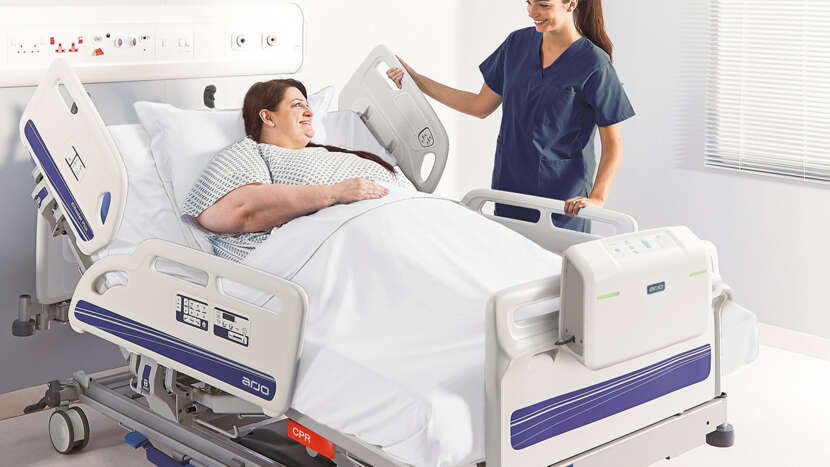
Auralis Plus provides an intelligent, dual-therapy system for plus size patients weighing up to 454 kg (1000 lbs), offering pressure redistributing properties that can play a significant role in increasing patient comfort while helping to reduce the risk of pressure injury.
This preventative therapy solution includes self-adjusting technology, a Heelguard® feature, and micro air-loss function to support the regulation of skin microclimate.
Designed to be compatible with the Citadel Plus bed frame, the Auralis Plus is easily width-adjustable to facilitate the safe and efficient transport of plus size patients.
Designed to be compatible with the Citadel Plus bed frame, the Auralis Plus is easily width-adjustable to facilitate the safe and efficient transport of plus size patients.
Premium fabric with welded seams
Enhanced coating is specifically designed to withstand longer exposure² to the active ingredients in cleaning chemicals and the abrasive action of repeated wipe down during routine cleaning process they endure.
Micro Air-Loss
The Micro Air-Loss function is designed to dehumidify the air surrounding the cells and aid in reducing heat build-up.
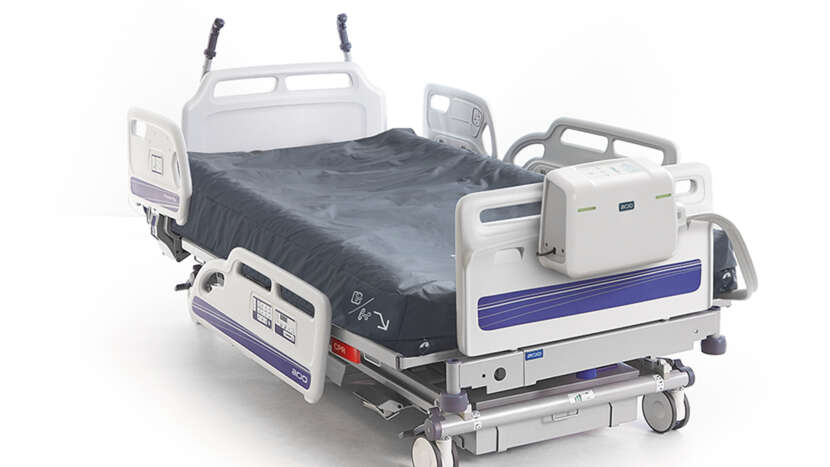
Width-adjustable mattress
Air Bolsters with control valves on each side of the mattress, are designed to enable simple and efficient width-adjustment, and can be removed for easier access and patient transfer, for example through doorways and in elevators for efficient patient transfer.
Heelguard® technology
Auralis incorporates six Heelguard® cells which help to redistribute pressure from the vulnerable heel area.
Auto-Firm mode
To assist with patient transfer and other nursing procedures, Auto-Firm mode maximises the air in the cells, providing a temporary stable surface.
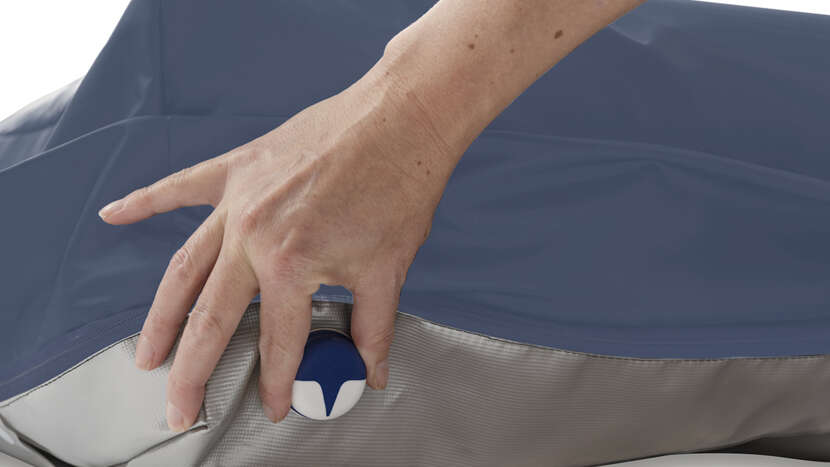
CPR Control
Designed for easy, one-handed operation, provides rapid deflation of the mattress to assist with resuscitation procedures.
Cable management
Stows the cable to help ensure no trailing power cable.
References:
1. The Impact of Cleaning Chemicals on Polyurethane Mattress Cover Materials and their Propensity for Physical Damage, Jo Milnes MA(Hons), MSc(Dist): Technical Manager, Dartex Coatings Limited, Acton Close, Long Eaton, Nottingham, NG10 1FZ. 2013
2. Design Verification Test Report 100082899 - Auralis Plus, November 2020
Enhanced coating is specifically designed to withstand longer exposure² to the active ingredients in cleaning chemicals and the abrasive action of repeated wipe down during routine cleaning process they endure.
Micro Air-Loss
The Micro Air-Loss function is designed to dehumidify the air surrounding the cells and aid in reducing heat build-up.
Width-adjustable mattress
Air Bolsters with control valves on each side of the mattress, are designed to enable simple and efficient width-adjustment, and can be removed for easier access and patient transfer, for example through doorways and in elevators for efficient patient transfer.
Heelguard® technology
Auralis incorporates six Heelguard® cells which help to redistribute pressure from the vulnerable heel area.
Auto-Firm mode
To assist with patient transfer and other nursing procedures, Auto-Firm mode maximises the air in the cells, providing a temporary stable surface.
CPR Control
Designed for easy, one-handed operation, provides rapid deflation of the mattress to assist with resuscitation procedures.
Cable management
Stows the cable to help ensure no trailing power cable.
References:
1. The Impact of Cleaning Chemicals on Polyurethane Mattress Cover Materials and their Propensity for Physical Damage, Jo Milnes MA(Hons), MSc(Dist): Technical Manager, Dartex Coatings Limited, Acton Close, Long Eaton, Nottingham, NG10 1FZ. 2013
2. Design Verification Test Report 100082899 - Auralis Plus, November 2020
| Pump | |
|---|---|
| Size (L x W x H) mm | 385 x 290 x 170 mm |
| Size (L x W x H) in | 15.2 x 11.4 x 9.7 in |
| Weight kg | 5.5 kg |
| Weight lb | 12.1 Ib |
| Case Material | ABS Plastic |
| Part number | 636XXX (XXX is determined by the type of mains lead fitted. Please refer to rear label on the pump for full part number) |
| SKIN IQ Accessory Coverlet | SIQCOBAS1UN* |
| SKIN IQ adapter part number | Power cable 636377 |
| Alternating cycle time | 10 minutes (Alternate 10 minutes, AutoFirm 15–30 minutes, CLP continuous) |
| Supply Voltage | 100–230 V |
| Power Input | 4–58 VA |
| IP Rating | IP21 |
| Sound output | 28.75 dBA** |
| Compatibility | The Auralis Pump is designed to operate with full range of Auralis therapeutic support surfaces. Please contact you local Arjo representative for more information. |
| Mattress Replacement | |
| Auralis Plus Mattress Replacement - Part Number 636B02 - size | 2140 x 1220 x 205 (84 x 48 x 8) |
| Auralis Plus (Width bolsters inflated) - Part Number 636B02 – size | 2140 x 1220 x 205 (84 x 48 x 8) |
| Auralis Plus (Width bolsters deflated) - Part Number 636B02 – size | 2140 x 860 x 205 (84 x 34 x 8) |
| Auralis Plus Long Mattress Extension Accessory - Part number: 636614 - size | 120 x 1220 x 205 (5 x 48 x 8) |
| Auralis Plus Short Mattress Extension Accessory - Part number: 636615 - size | 120 x 860 x 205 (5 x 34 x 8) |
| Weight kg (lb) (includes the transport bag of 0.46 kg) | 16 kg (35.3 lb) |
| CPR | Rapid deflate within 15 seconds at head end |
| Maximum patient weight | 454 kg (1000 lbs) |
Explore this product
Filter
Filter
Auralis Plus Brochure
Type: Brochure
Auralis Plus Specification Sheet
Type: Product specifications sheet
Auralis Instructions for use
Type: Instructions for use (IFU)
Auralis Quick Reference Guide - Cleaning and disinfection (A4, Portrait, colour)
Type: Quick Reference Guide (QRG)
Auralis Quick reference guide - Controls (A4, Portrait, Colour)
Type: Quick Reference Guide (QRG)
Auralis Quick reference guide - Use (A4, Portrait, Colour)
Type: Quick Reference Guide (QRG)
Clinical Performance Testing Auralis Plus
Type: Clinical summary and/or focus document
The Science Behind the Support Surface
Type: Clinical evidence brochure
EPUAP 2024 Poster Dialling Down the Pressure
Type: Scientific poster or presentation
Auralis Plus Brochure
Type: Brochure
Auralis Plus Specification Sheet
Type: Product specifications sheet
Auralis Instructions for use
Type: Instructions for use (IFU)
Auralis Quick Reference Guide - Cleaning and disinfection (A4, Portrait, colour)
Type: Quick Reference Guide (QRG)
Auralis Quick reference guide - Controls (A4, Portrait, Colour)
Type: Quick Reference Guide (QRG)
Auralis Quick reference guide - Use (A4, Portrait, Colour)
Type: Quick Reference Guide (QRG)
Clinical Performance Testing Auralis Plus
Type: Clinical summary and/or focus document
The Science Behind the Support Surface
Type: Clinical evidence brochure
EPUAP 2024 Poster Dialling Down the Pressure
Type: Scientific poster or presentation