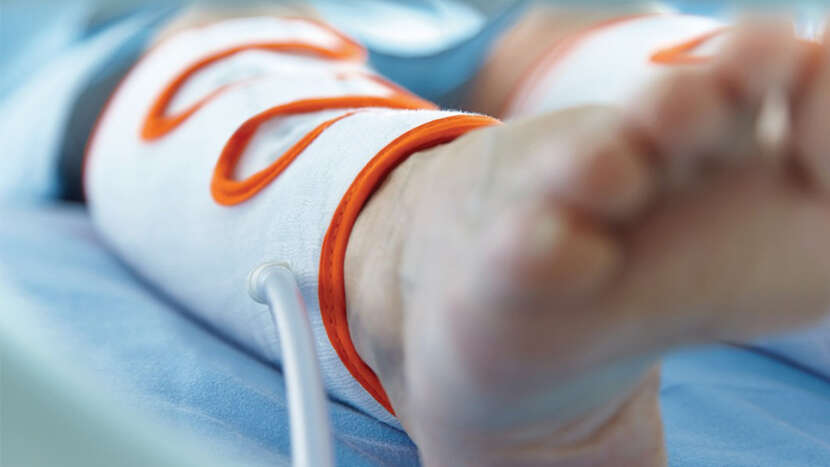
Flowtron Tri Pulse
Sequential compression designed for optimal comfort
Built on a sequential (multi-chamber bladder) compression philosophy,Flowtron Tri Pulse is a premium garment range designed for optimal anatomical fit and enhanced patient comfort
PROVEN PREMIUM COMFORT AND DESIGNWith comfortable, premium fabrics making the patient more inclined to wear the garments during therapy, Flowtron addresses the core challenge of comfort in VTE prevention. Proven comfortable, Flowtron garments promote effective prevention and improved patient outcome.¹⁻³
USING FLOWTRON
The Flowtron IPC system is suitable for DVT prophlyaxis in acute care settings, in the pre-, intra- and post-surgery phases, as a sole agent or when combined with a pharmacological agent.
CONTRAINDICATIONS
IPC should NOT be used in the following conditions:
Severe arteriosclerosis or other ischaemic vascular diseases
Severe congestive heart failure or any condition where an increase of fluid to the heart may be detrimental
Known or suspected acute deep vein thrombosis, thrombophlebitis or pulmonary embolism
Any local condition in which garments would interfere including gangrene, recent skin graft, dermatitis, on untreated, infected leg wounds.
REFERENCES:
1. Busby J, Holst K, Hansson K. User feedback on the Flowtron® ACS900 pump and Tri Pulse garment range. Arjo Whitepaper. June 2021. Arjo.A00491.1.0.INT.EN.
2. Arjo Independent Test Data on File. Tri Pulse: water vapour resistance, thermal resistance (single plate method), drying time, liquid wicking rate and water vapour permeability testing. September 2019. Test report E-008677/C.
3. Pagella P, Cipolle M, Sacco E et al. A randomised trial to evaluate compliance in terms of patient comfort and satisfaction of two pneumatic compression devices. Orthop Nurs. 2007; 26(3):169-74.
References
1. Arjo Independent Test Data on File. Tri Pulse: water vapour resistance, thermal resistance (single plate method), drying time, liquid wicking rate and water vapour permeability testing. September 2019. Test report E-008677/C.
2. Kucher N, Koo S, Quiroz R et al. Electronic alerts to prevent venous thromboembolism among hospitalized patients. N Engl J Med. 2005; 352:969-977.
3. Pagella P, Cipolle M, Sacco E et al. A randomised trial to evaluate compliance in terms of patient comfort and satisfaction of two pneumatic compression devices. Orthop Nurs. 2007; 26(3):169-74.
ANATOMICAL SHAPE
Sequential garment range designed for optimal anatomical fit and enhanced patient comfort
PREMIUM MATERIALS
Advanced Airflow Light fabric keeps the skin cool and dry by preventing heat and moisture build-up1
PATENTED DESIGN
Garment designed to follow the natural curve of the leg, with patented wing-shaped bladder wrapping around the calf
SINGLE AIR INLET TUBE
Single air inlet tube positioned anteriorly on the leg away from the bony prominence, designed to help reduce the risk of pressure injuries and to promote safety with less tubing around the patient2
VELCRO® FASTENERS
· Simple and robust Velcro® closures helping to promote effective therapy by providing a secure and snug fit2
APPLICATION GUIDE
· Clear visual instructions printed on the garment for ease of use and safety in application3
REFERENCES:
1. Arjo Independent Test Data on File. Tri Pulse: water vapour resistance, thermal resistance (single plate method), drying time, liquid wicking rate and water vapour permeability testing. September 2019. Test report E-008677/C.
2. Busby J, Holst K, Hansson K. User feedback on the Flowtron® ACS900 pump and Tri Pulse garment range. Arjo Whitepaper. June 2021. Arjo.A00491.1.0.INT.EN.
3. Arjo Data on File: Formative Evaluation Report 100082820. December 2019.
Sequential garment range designed for optimal anatomical fit and enhanced patient comfort
PREMIUM MATERIALS
Advanced Airflow Light fabric keeps the skin cool and dry by preventing heat and moisture build-up1
PATENTED DESIGN
Garment designed to follow the natural curve of the leg, with patented wing-shaped bladder wrapping around the calf
SINGLE AIR INLET TUBE
Single air inlet tube positioned anteriorly on the leg away from the bony prominence, designed to help reduce the risk of pressure injuries and to promote safety with less tubing around the patient2
VELCRO® FASTENERS
· Simple and robust Velcro® closures helping to promote effective therapy by providing a secure and snug fit2
APPLICATION GUIDE
· Clear visual instructions printed on the garment for ease of use and safety in application3
REFERENCES:
1. Arjo Independent Test Data on File. Tri Pulse: water vapour resistance, thermal resistance (single plate method), drying time, liquid wicking rate and water vapour permeability testing. September 2019. Test report E-008677/C.
2. Busby J, Holst K, Hansson K. User feedback on the Flowtron® ACS900 pump and Tri Pulse garment range. Arjo Whitepaper. June 2021. Arjo.A00491.1.0.INT.EN.
3. Arjo Data on File: Formative Evaluation Report 100082820. December 2019.
| Ordering Information | |
|---|---|
| Code | Product Description | Quantity per Carton |
| TRP10 | Regular Calf Garment (up to 43cm/17" calf circumference) | 10 pairs |
| TRP20 | Large Calf Garment (up to 58cm/23" calf circumference) | 10 pairs |
| TRP60L | Bariatric Calf Garment (up to 81cm/32" calf circumference) | 10 pairs |
| TRP30 | Regular Thigh Garment (up to 71cm/28" calf circumference) | 10 pairs |
| TRP40 | Large Thigh Garment (up to 89cm/35" calf circumference) | 10 pairs |
Explore this product
Filter
Filter
VTE Patient Leaflet
Type: Brochure
Flowtron Tri Pulse Specification Sheet
Type: Product specifications sheet
Flowtron garments - Instructions for use
Type: Instructions for use (IFU)
Garment Sizing and Application Guide
Type: Sales support material
Flowtron Tri Pulse Garment Comfort Whitepaper
Type: Whitepaper
IPC and Compliance Monitoring Whitepaper 1.1
Type: Whitepaper
VTE Patient Leaflet
Type: Brochure
Flowtron Tri Pulse Specification Sheet
Type: Product specifications sheet
Garment Sizing and Application Guide
Type: Sales support material
Flowtron Tri Pulse Garment Comfort Whitepaper
Type: Whitepaper
IPC and Compliance Monitoring Whitepaper 1.1
Type: Whitepaper
Flowtron garments - Instructions for use
Type: Instructions for use (IFU)