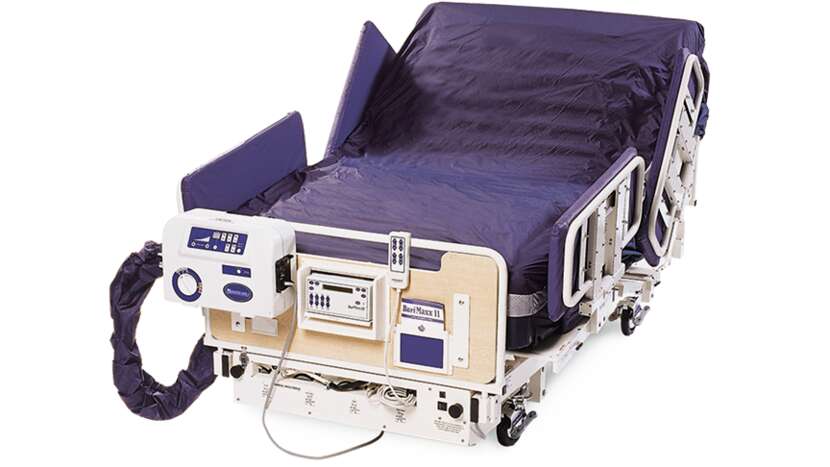
MaxxAir ETS
The MaxxAir ETS Mattress Replacement System (MRS) provides skin management therapies and enables safe patient handling for patients weighing up to 454 kg (1,000 lbs).
Designed for use with the BariMaxx™ II Therapy System and the BariMaxx™ II with Power Drive System.With Low Air Loss (LAL) therapy, Continuous Lateral Rotational Therapy (CLRT), GORE® medical fabric and a turn assist feature, the MaxxAir ETSMRS is designed for use with the BariMaxx II Therapy System. The MaxxAir ETSMRS helps maintain skin integrity and improves safe patient handling, thereby reducing the risks of musculoskeletal injuries associated with bariatric care.
Indications
*Prevention and treatment of pressure injuries.
*Patients who are difficult to turn.
*Patients using a static mattress that would benefit from a pressure redistribution surface.
*Patients whose body weight and size pose a significant risk or care management issue to the patient or staff during routine nursing care procedures.
*Patients weighing up to 454 kg (1,000 lbs) (including accessories).
Contraindications
*Unstable cervical, thoracic and/or lumbar fractures.
*Cervical or skeletal traction.
*Patients with a total weight in excess of 454 kg (1,000 lbs) (including accessories).
*Please refer to product labelling and instructions for use for risks, precautions, warnings and detailed operating instructions prior to use.
Continuous Lateral Rotation Therapy (CLRT) offloads pressure by constantly changing the pressure points.
Low Air Loss (LAL) therapy provides airflow to help maintain skin temperatures and control and manage moisture.
GORE® medical fabric offers excellent moisture vapour permeability to create the optimum skin microclimate while serving as an effective barrier against fluid and bacteria.
Full body turn assist, up to 30°, helps facilitate resident/patient care by automatically repositioning and turning residents/patients.
Mattress expandability from 91 cm to 107 cm to 122 cm (36” to 42” to 48”) allows customisation of width for resident/patient comfort and safety while maintaining dignity and enabling easy transfers.
Rapid, convenient CPR deflation.
Easy-to-use controls at the foot of the bed for simplified nursing care.
454 kg (1,000 lbs) weight capacity accommodates high resident/patient weight in both static and turning positions.
Low Air Loss (LAL) therapy provides airflow to help maintain skin temperatures and control and manage moisture.
GORE® medical fabric offers excellent moisture vapour permeability to create the optimum skin microclimate while serving as an effective barrier against fluid and bacteria.
Full body turn assist, up to 30°, helps facilitate resident/patient care by automatically repositioning and turning residents/patients.
Mattress expandability from 91 cm to 107 cm to 122 cm (36” to 42” to 48”) allows customisation of width for resident/patient comfort and safety while maintaining dignity and enabling easy transfers.
Rapid, convenient CPR deflation.
Easy-to-use controls at the foot of the bed for simplified nursing care.
454 kg (1,000 lbs) weight capacity accommodates high resident/patient weight in both static and turning positions.
| Dimensions | |
|---|---|
| Mattress A (L x W x H) mm | 2030 x 910 x 250 mm |
| Mattress A (L x W x H) in | 80 x 36 x 25 in |
| Mattress A Weight kg | 22 kg |
| Mattress A Weight lb | 48 lb |
| Mattress B (L x W x H) mm | 2030 x 1070 x 250 mm |
| Mattress B (L x W x H) in | 80 x 42 x 10 in |
| Mattress B Weight kg | 22 kg |
| Mattress B Weight lb | 48 lb |
| Mattress C (L x W x H) mm | 2030 x 1220 x 250 mm |
| Mattress C (L x W x H) in | 80 x 48 x 10 in |
| Mattress C Weight kg | 22 kg |
| Mattress C Weight lb | 48 lb |
| Cell Material | Polyurethane |
| Pump | |
| Size (L x W x H) mm | 17 x 42 x 27 mm |
| Size (L x W x H) in | 6.7 x 16.5 x 10.5 in |
| Weight kg | 5,7 kg |
| Weight lb | 12,5 lb |
| Mode of Operation | Continuous |
| Electrical Rating | 60 Hz |
| Operating Cycle Time | 3-30 minutes |
| Shock Protection | Class II, Type BF |
| Ingress Protection | IPX1 |
| Power In | 115 Volt, 5 Amp |
| Standards | UL60601-1 |
Explore this product
Filter
Filter
MaxxAir ETS Instructions for use Mattress Replacement System User's Guide
Type: Instructions for use (IFU)
MaxxAir ETS Quick reference guide - Funcstions (A4, Portrait, Colour)
Type: Quick Reference Guide (QRG)
MaxxAir ETS Quick reference guide - Use (A4, Portrait, Colour)
Type: Quick Reference Guide (QRG)
MaxxAir ETS Instructions for use Mattress Replacement System User's Guide
Type: Instructions for use (IFU)
MaxxAir ETS Quick reference guide - Funcstions (A4, Portrait, Colour)
Type: Quick Reference Guide (QRG)
MaxxAir ETS Quick reference guide - Use (A4, Portrait, Colour)
Type: Quick Reference Guide (QRG)