Nimbus 4
Alternating Pressure System
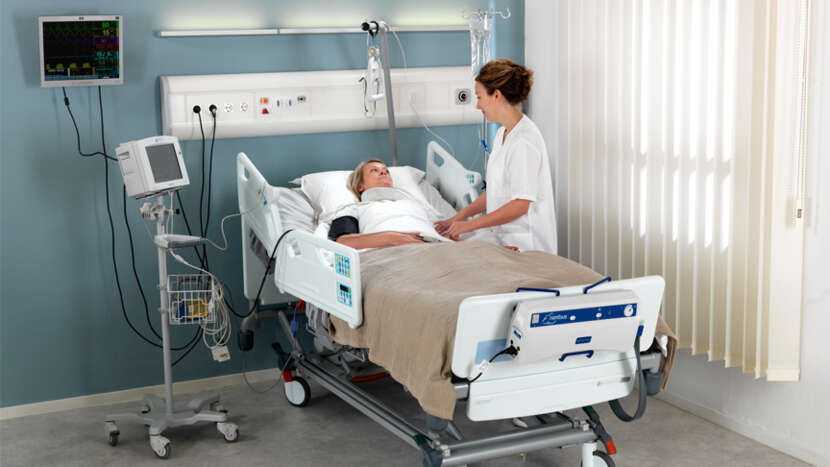
The Nimbus 4 system combines automatic adjustment of cell pressures, Heelguard and Wound Valve Technology providing effective pressure redistribution and enhanced solutions to a wide range of patient management issues.
In Clinical Use for more than 20 years Nimbus Alternating Mattress Replacement Systems are proven to be clinically and cost effective pressure redistributing mattress replacement systems used across all care environments.¹⁻⁸References:
1. Ward C, Wubbels M, Schembri L (2012) Using Complete Pressure Off-loading and Advanced Wound Care to Treat a Complex Sacral Pressure Ulcer. Poster Presentation
2. Clancy J (2011) Winning the war against pressure ulcers. Poster presentation
3. Malbrain M, Hendriks B, Wijnands P et al (2010) A pilot randomised controlled trial comparing reactive air and active alternating pressure mattresses in the prevention and treatment of pressure ulcers among medical ICU patients. Journal of Tissue Viability 19(1):7-15
4. Finnegan MJ (2008). Comparing the effectiveness of a specialised alternating air pressure mattress replacement system and an air fluidised integrated bed in the management of post- operative flap patients. A randomised controlled study. Journal of Tissue Viability 17(1): February 2008
5. Russell L, Reynolds TM (2000) Randomised controlled trial of two pressure relieving systems. Journal of Wound Care 9(2):52-55
6. Evans D, Land L, Geary A (2000). A clinical evaluation of the Nimbus 3 alternating pressure mattress replacement system. Journal of Wound Care 9(4):181-186
7. Land L, Evans D, Geary A et al (2000) A clinical evaluation of an alternating pressure mattress replacement system in hospital and residential care settings. Journal of Tissue Viability 10(1): 6-11
8. Phillips L (2010) Nimbus range of pressure redistributing mattresses. Wounds UK, 2010, 6 (2): 116-122
Audible and visual alarms
• The pump includes audible and visual alarms, activating only when genuine intervention is needed for Power Fail, High or Low Pressure and Pump Fault conditions.
Comfort control
• For additional patient comfort, minor adjustments to inflation pressures can be made using a comfort control dial, without affecting therapeutic performance.¹⁶
Infection prevention
• 3 stage filtration provided by the bio-filter ensures only clean air is passed into the mattress.
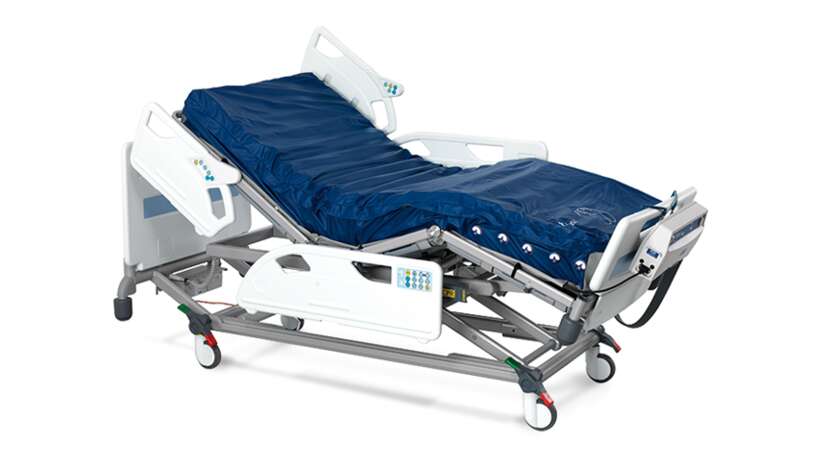
Nimbus range mattress features
Cover
• Vapour permeable and water resistant 2-way stretch cover offers pressure redistribution and comfort. This loose fitting top cover is attached to the mattress base by concealed zips to reduce ingress of unwanted fluids. The cover is also flame retardant, easy to clean and can be completely removed for laundering.
Auto-matt sensor pad
• The Auto-Matt sensor pad is designed to adjust cell pressures according to patient weight and positioning on the mattress.
Anti-sink mattress design
• The mattress is made up of 19 deep "figure of 8" designed cells to support patients up to 250 kg, providing active pressure redistribution for all patient types and all categories⁹ of tissue damage.
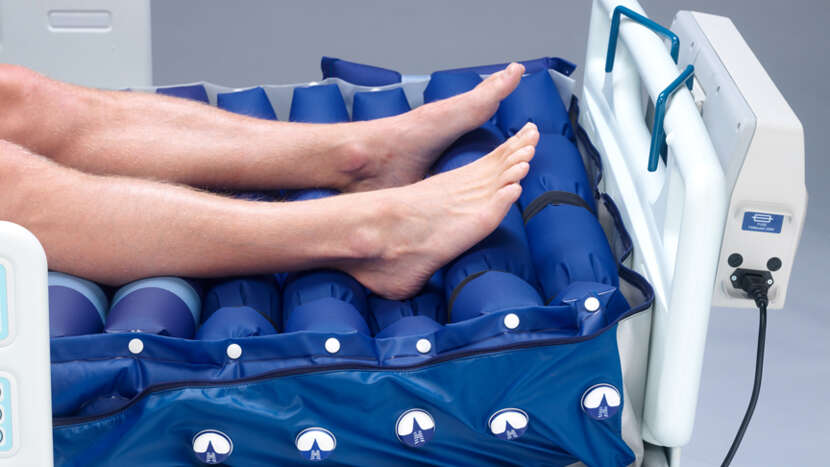
Wound valve technology
• 5 heel cells incorporate Wound Valve technology, allowing the carer to permanently off-load ("float") the vulnerable heel, for those patients with limb ischaemia or existing wounds.
Cable management
• Reduces risk of injury to patient and/or carer by removing trailing cables.
Transport facility
• Seals air in the mattress for up to 12 hours, providing a uniform stable surface for patient transportation, moving and handling, nursing procedures and during periods of power failure.
Heelguard
• Dedicated mattress zone where bottom 5 cells provide maximum pressure redistribution through use of unique "power down" straps, keeping pressure at the vulnerable heel lower for longer (Nimbus 4).
CPR facility
• A quick release, single handed operation, the CPR facility enables rapid air deflation for emergencies, short term physiotherapy, moving and handling or storage.
References:
9. National Pressure Ulcer Advisory Panel, European Pressure Ulcer Advisory Panel and Pan Pacific Pressure Injury Alliance. Prevention and Treatment of pressure ulcers: Clinical practice guideline. Emily Haesler (Ed). Cambridge Media: Osbourne Park, Western Australia; 2014
16. Arjo Data on File. Product Validation Test Report 10-100037760
| Dimensions | |
|---|---|
| Mattress A Name | Standard |
| Mattress A (L x W x H) mm | 2085 x 890 x 215 mm |
| Mattress A (L x W x H) in | 82 x 35 x 8.5 in |
| Mattress A Weight kg | 15,5 kg |
| Mattress B Name | Narrow |
| Mattress B (L x W x H) mm | 2085 x 800 x 215 mm |
| Mattress B (L x W x H) in | 82 x 31.5 x 8.5 in |
| Mattress B Weight kg | 14,3 kg |
| Mattress B Weight lb | 31,5 lb |
| Maximum patient weight kg | 250 kg |
| Maximum patient weight lb | 550 lb |
| Cell Material | Polyurethane |
| Pump | |
| Size (L x W x H) mm | 508 x 220 x 100 mm |
| Size (L x W x H) in | 20 x 8.75 x 4 in |
| Weight kg | 5,7 kg |
| Weight lb | 12,5 lb |
| Mode of Operation | Continuous |
| Electrical Rating | 35VA |
| Operating Cycle Time | 10 minutes |
| Shock Protection | Class II Type BF |
| Ingress Protection | IP21 |
| Power In | 230 VAC, 50Hz |
| Standards | EN60601-1, IEC60601-1 |
Explore this product
Type: Sales | Specifications sheet / Flyer
Type: Sales | Leave behind / Brochure
Type: Instructions for use (IFU)
Type: Instructions for use Abstract (IFU Abstract)
Type: Instructions for use (IFU)
Type: Quick Reference Guide (QRG)
Type: Quick Reference Guide (QRG)
Type: Quick Reference Guide (QRG)
Type: Sales | Leave behind / Brochure
Type: Sales | Specifications sheet / Flyer
Type: Sales | Leave behind / Brochure
Type: Sales | Leave behind / Brochure
Type: Clinical | Clinical evidence brochure
Type: Sales | Specifications sheet / Flyer
Type: Clinical | Clinical evidence brochure
Type: Sales | Specifications sheet / Flyer
Type: Sales | Specifications sheet / Flyer
Type: Sales | Leave behind / Brochure
Type: Sales | Leave behind / Brochure
Type: Sales | Specifications sheet / Flyer
Type: Sales | Leave behind / Brochure
Type: Sales | Leave behind / Brochure
Type: Clinical | Clinical evidence brochure
Type: Sales | Specifications sheet / Flyer
Type: Clinical | Clinical evidence brochure
Type: Sales | Specifications sheet / Flyer
Type: Instructions for use (IFU)
Type: Instructions for use Abstract (IFU Abstract)
Type: Instructions for use (IFU)
Type: Quick Reference Guide (QRG)
Type: Quick Reference Guide (QRG)
Type: Quick Reference Guide (QRG)